- "Sodium forms a +1 ion because it wants to have a noble gas electron configuration."
- "Plants evolve because it gives them a higher chance of survival."
- "My truck wants a lot of gas when rock crawling."
Each of these statements imply that inanimate objects have desires. They don't. They respond to energy and to entropy to minimizing the former and to maximizing the latter. And it's not even their own energy and entropy to which they respond, it's the energy and entropy of the entire world that is driving change.
The most important equation in the universe is Gibbs law,
ΔG = ΔH - TΔS
ΔG is the Gibbs Free Energy, the energy free to do work, or the energy free to drive the system, which is a description of the current state of affairs and the predicted future state of affairs, such that
ΔG = Gfinal - Ginitial
If this is negative the system will move to completion. ΔG is not directly measurable, so we use Gibbs law to use the two measurable quantities, Enthalpy, ΔH, and Entropy, ΔS, to find it.
Enthalpy, ΔH, is the heat given off by a process. The fun thing about heat is that all forms of energy, heat, light, pressure, sound, falling, will end up as heat, so this term catches all the energies involved.
Entropy, ΔS, is harder to define. This is a measure of disorder in the world, and it's driven by Vegas odds. How many different ways can the current state of affairs exist, and how many ways can a future state of affairs exist. This is a statistical calculation, and when atoms are involved (as they always are) creates massive numbers of different ways, down to the level of where each atoms is located and how it is vibrating. The fun thing about entropy is that it always goes up. So ΔS is always a positive number. If a proposed system change generates a negative ΔS, that change can never happen. T is the absolute temperature in Kelvin, so the higher the temperature, the more influence entropy has.
All inanimate things respond to maximize the negative value of ΔG. Most animate things do too. Sometimes it seems like they don't, but they really do. Jogging for fun, for example, seems like that expends a huge amount of energy to build up your muscles, but it's really expending a huge amount of heat (ΔH is very negative) and turning a lot of dissolved sugar into carbon dioxide gas, making ΔS hugely positive.
It's the world that finds this minimum energy/maximum entropy position, not the lone atoms/plant/whatever. When an atoms ionizes, that electron has to go somewhere, so there must be another atoms to accept that electron, and in the transfer, energy must drop overall, and entropy must rise overall.
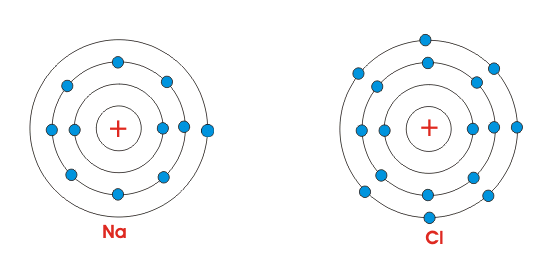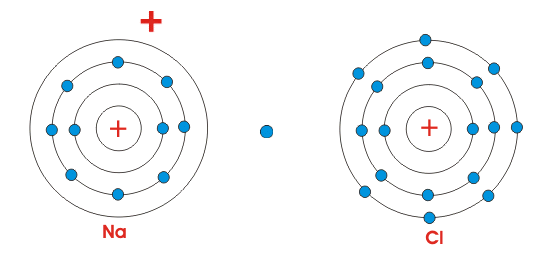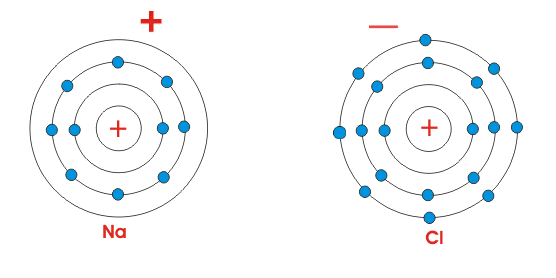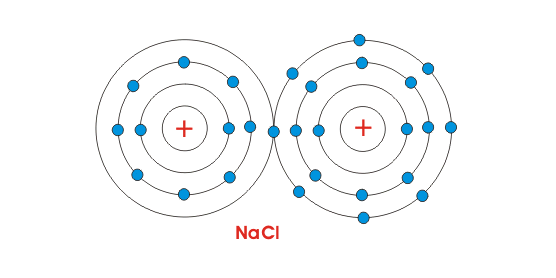
So when we examine sodium, it has no wants and desires; the world went to lower energy/higher entropy when the electron transfer happened, so it happened.
The anthromorphization of atoms and plants and anything else is because we don't understand the system or are too lazy to try.
And there is harm in it. By attributing an event to the wrong cause, we are more likely to mistake our observations because our preconceptions color our observations so completely. This was a strong factor in the longevity of alchemy; alchemists thought they were seeing one thing, when something entirely different was happening. They mistook reality for a sort of fantasy world they had read about. When they did a reaction in an iron pan, and the product was green (from iron sulfate or iron chloride), it never occurred to them that some of the iron had reacted; they thought it was just the next step in the synthesis of gold because that's how previous alchemists had described it and that the iron pan was inert. So they plugged along on alchemy and missed reality, so they missed all the observations that would have taken them toward progress. While alchemy and the theories behind it was alive in the minds of alchemists there was no scientific progress. Zero.
It's quite a thing, stopping all progress.
I'm continually on the watch for that happening in our science. And there is a lot of it, if you go back to read my last post on alchemy.