There is no date for the death of alchemy. What we see is a long series of changes to the four elements until the early chemists arrive at a new way of looking at nature.
After the mercury/sulfur/salt theory of Paracelsus, (still alchemy), the first major change is the Phlogiston theory of Stahl, 1717, when Newton and most alchemists were still practicing alchemy. Phlogiston is the Fire from Empedocles, a substance emanating from the sun, which gathers into wood, and when heated, the phlogiston is released. Metals also contained a great amount of phlogiston, and would burn when heated sufficiently to drive it out.
Joseph Priestly experimented in the 1770's with oxygen gas, which he called "dephogisticated air" because, having no phlogiston in it, would accept phlogiston from almost any source. He also played with carbon dioxide from a nearby brewery, which did not support flame, which he called "phlogisticated air" because it was already saturated with phlogiston and would accept no more from a flame. His efforts meant very little to the practicing alchemists. But they meant a lot to a French tax collector named Antoine-Laurent Lavoisier.

Joseph Priestly
Lavoisier was good at numbers and accounting, which made him very wealthy working for a Paris tax collector. He married the boss's daughter (Marie-Anne, which turned out to be the best thing he could have done; she was amazing), got into minor politics, made gunpowder for the government (and for the American Revolution), made government fertilizer for French farms, and finally, semi-retired, wanted to figure out this alchemy thing. But Lavoisier's approach was not like the alchemists in any way. He did not follow the sequence of colors, nor follow known descriptions of what he should see; he bought the best balance he could get, from a London instrument maker, and applied accounting to his measurements.

Marie-Anne Lavoisier and Antoine-Laurent Lavoisier, painted by David, who mentored Marie-Anne in art.
At a social dinner Marie-Anne sat between Lavoisier and Joseph Priestly. She knew English and translated for Lavoisier as Priestly talked at length about dephlogisticated air. Marie-Anne was beautiful and I think Priestly got a little carried away with himself impressing her, so Lavoisier walked away with a huge amount of information about the gas he would call "oxygen."
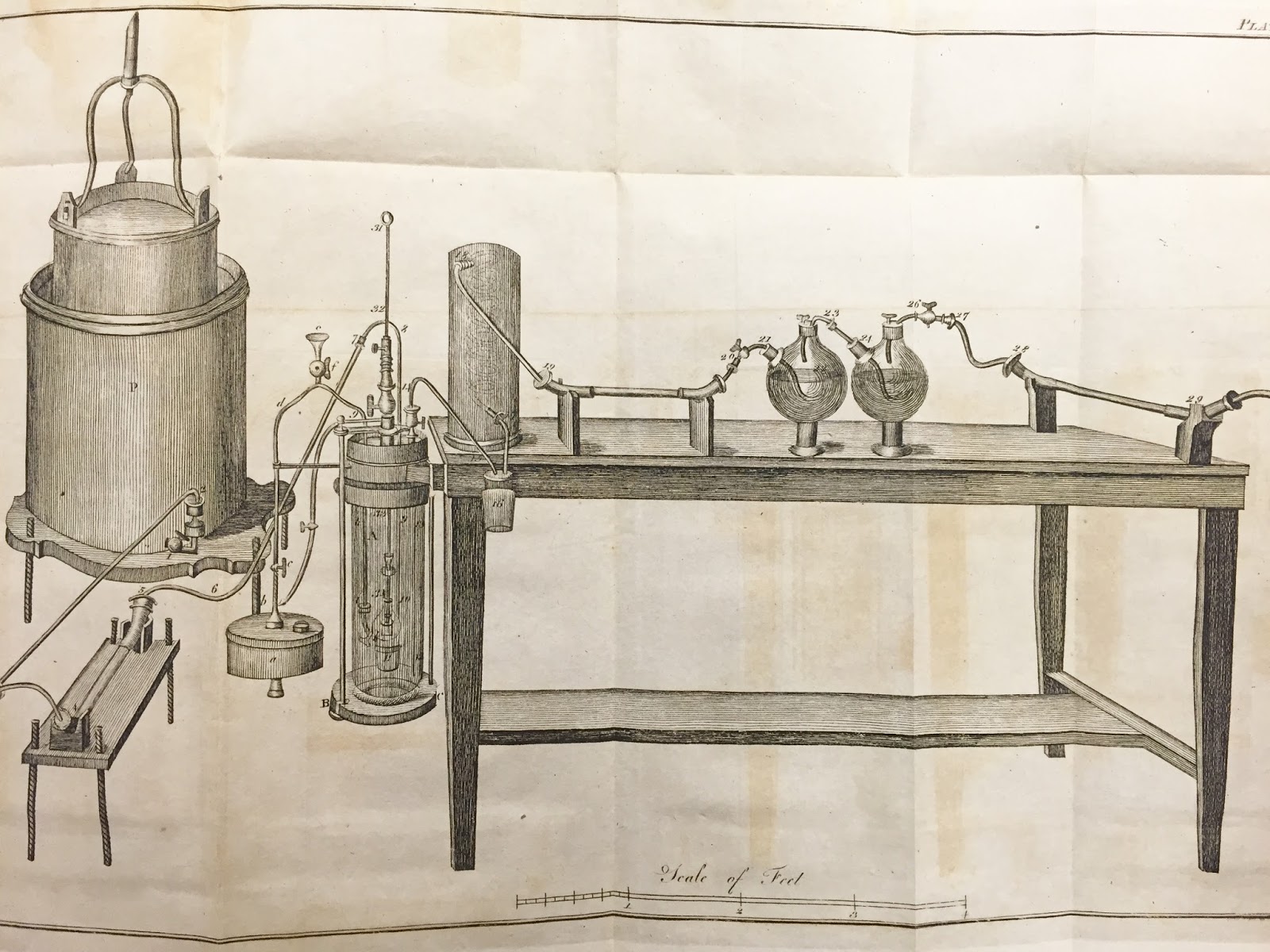
Lavoisier's experiments were of this type: start with a weighed sample of metal and with oxygen, and let them react in a closed vessel. Weigh them again (the weight was the same), then using a higher heat, separate them again (it was the same weight again). From this he got the second law in chemistry, the Law of Conservation of Mass. He also learned a lot about what things can be broken into multiple other substances, and which could not, leaning hard into a new theory of elements, but not stating it well.
Lavoisier then wrote a book on how to do chemistry in 1779, which his wife illustrated (she was a student of the painter David, who did the portrait of them seen above). The illustrations were beautiful, and the book was quickly translated into English and sold fast.


What Lavoisier had done would later be called a paradigm shift, a change in the was of doing and thinking about alchemy that the old alchemists would not have realized was even a thing.
After Lavoisier there were still many practicing alchemists. There still are. But the new chemistry, the science of measurement and of experiments, was alive and well.
Is there a death date? Maybe, but it's not the death of alchemy that marks the date: it's the birth of something better, the reemergence of atomic theory by John Dalton, 1803, and his publication, 1808. More on that in my new blog series, Early Chemistry.
____________________________________
So what of this blog series? I'll update it still, to fill in the many blanks I left open when I could not find good quotations from the many alchemists I skipped over. And there were quite a few. I'm not sure how I'll number them, but maybe 'Alchemy 35.5" or something, to maintain the sequence.
And I'll start a new series, Early Chemistry. I'm not as well read there, so a year of study or so will be needed. Maybe I'll find the topic too big to do. Maybe it's too small and I'll need to extend it to Early Science. Dunno yet.