Science magazine reports that a marine biologist at the University of Delaware has committed scientific fraud by making up data supporting the idea that increasing the carbon dioxide concentration in the atmosphere will increase the pH of ocean waters enough to affect the behaviors of marine wildlife. This is a big deal, because so many subsequent reports "confirm" this, in dozens of papers, while much replicating research did not. And all these reports made it into the press because they confirmed the climate change story/myth/theory. And now it's all being knocked down. The coral and the fish, it seems, are quite safe.

When these reports first surfaced I talked about them with my students, my freshman chemistry students, about the possibility that CO2 could influence the pH. Here is the problem with that idea: The carbon dioxide is part of the equilibrium. That means it can't, by definition, have any major effect whatever on the pH. Here are the reactions, with associated equilibrium constants, starting with a Henry's Law calculation at 400 ppm CO2:
(1) CO2(g) ⇄ CO2(aq) Keq = very small; [CO2] = 400 x 10-6 atm/29.41 atm M-1 = 1.36 x 10-5 M @ 400 ppm CO2
(2) CO2(aq) + H2O(l) ⇄ H2CO3(aq) Kc = 1.6 x 10-3
(3) H2CO3(aq) ⇄ H+(aq) + HCO3-(aq) Ka1 = 2.5 x 10-4
It is here that most climate change people stop the calculation, perhaps because at this point, and with sufficient ignorance, it appears that more CO2 means more H+ and more acidity. By combining the two equilibria into an overall equilibrium (Kc = 4.0 x 10-7) and using the concentration of CO2(aq), they get a pH addition of +1.3 x 10-5 M H+. This is a pH of 4.9. And this acidification would have been happening ever since there was carbon dioxide in the atmosphere, which is the entire life of the planet! The oceans should be totally acidified by now.
The pH of the ocean is 8.1. Which is basic. Not acidic. And adding a pH of 4.9 would make the ocean far more acidic. The failure is when they don't ask: why is the ocean alkaline? Here's why:
(4) CaCO3(s) ⇄ Ca2+(aq) + CO32-(aq) Ksp = 6 x 10-9
(5) CO32-(aq) + H2O(l) ⇄ HCO3-(aq) + OH-(aq) Kb2 = 1.8 x 10-4
Carbon dioxide acidifies, and calcium carbonate creates basic conditions, but in reality they are in equilibrium with each other, to complete the cycle with the reaction of H+ and OH- to make water:
(6) H+(aq) + OH-(aq) ⇄ H2O(l) 1/Kw = 1 x 1014
Add all the reactions together and form the equilibrium expression for the system:
(7) CaCO3(s) + CO2(aq) + H2O(l) ⇄ 2 HCO3-(aq) + Ca2+(aq) Kc = 4.3 x 10-5
with [CaCO3(s)] = 1, [CO2(aq)] = 1.4 x 10-5 M, [H2O(l)] = 1, making the concentration of the hydrogen carbonate ion dependent system dependent on dissolved carbon dioxide and calcium ion concentrations. And temperature (I haven't tried to find all that data; I've used the 25 C values):
(8) Kc = [HCO3-]2[Ca2+]/[CO2] = 4.3 x 10-5
This ignores calcium ion sinks like CaOH+, but I'll explain why in a bit.
So there is a constant source of carbon dioxide (the atmosphere), and a constant source of calcium carbonate (all the shells of living wildlife in the ocean, and their dead skeletons), and both of those also work as sinks of CO2 and CO32-. Both are in equilibrium with species which are not in the pH equilibrium (the gaseous carbon dioxide and the solid calcium carbonate). The calcium ion concentration, it seems, is the most sensitive part of the equilibrium, but it is always a very constant concentration (close to 10-4 M). So why do I discount the possible variability of calcium ion concentrations? Because it is being actively incorporated into the skeletons and shells of marine life. Active incorporation means they can drive the system toward non-equilibrium by using chemical energy, absorbing calcium in all it's soluble forms, including the CaOH- ion. Sea-life is dependent on that calcium to build shells and marine life spends a lot of energy gathering it up. The main chemistry of the ocean is all about the calcium ion; it's the limiting reagent for marine life (well, that and phosphate ion). If life is present, the calcium concentration will be driven down to a value about 10-4 M, when more calcium carbonate dissolves, releasing hydroxide ion, which helps more carbon dioxide dissolve to provide more hydrogen carbonate ion. Ocean pH levels are all about the calcium ion concentrations. Here is a graph of the equilibrium system, taken from a soil science lecture here:
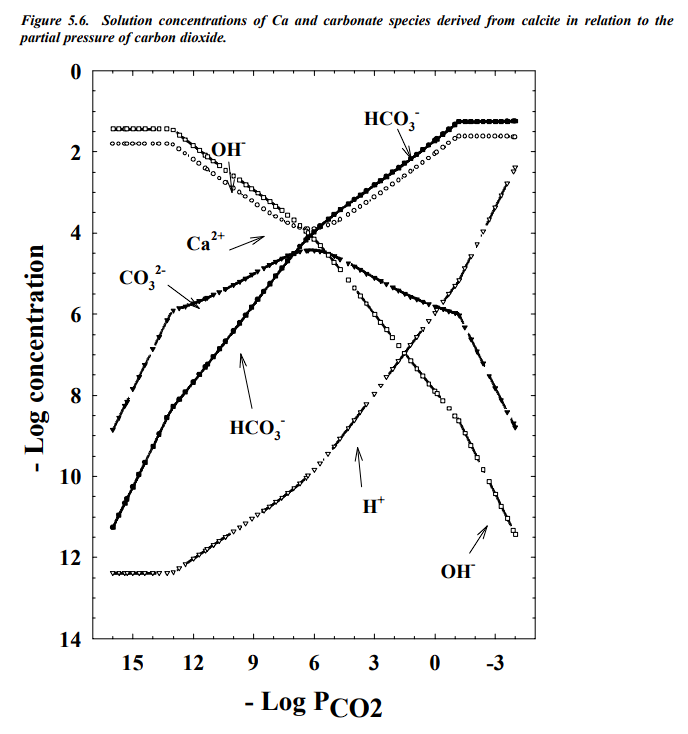
See that dip in the Ca2+ concentrations, the open circles? That dip is why the ocean pH doesn't change. Marine animals actively absorb the calcium ions to minimize its concentration, then the rest of the equilibrium system responds. When CO2 is more abundant, so are ocean plants, feeding the animals which grow, so calcium is absorbed, more calcium carbonate dissolves, and we are back to equilibrium, pH 8.1. Adding calcium ions to the water won't help, it just shifts the equilibrium momentarily to feed the animals until the ion concentrations drop back down or by precipitation of the excess calcium ions, pH 8.1. The animals are in charge of this system, so long as they absorb the calcium ions. Do anything to shift the pH, and more marine life is the consequence. It's a beautiful system.
I didn't have this graph when I explained it to my students, but I knew this is what water chemistry does with carbon dioxide and calcium carbonate both present. It's right there in the equilibrium reactions.
So the big question: why did none of these reviewers, nor the reviewers for all the dozens of papers which followed these, not pick up on this obvious consequence of equilibrium? I am utterly baffled as to why.
I've seen similar behavior in smaller studies, when spectroscopic peaks were picked from a graph that were obviously noise (papers later withdrawn from JACS and Science). Why did no one pick up on the wrongness of the interpretation? Peer review isn't this difficult. My suspicion: fraud is accepted in academia. "I'll let your paper slide into print if you let mine." It is not a beautiful system.
Once again, rely on the data, ignore the theory. Robert Boyle was right. Every damn time.